Dose Planning for COVID-19 Patients
In a recent study it was reported that radiation may reduce inflammation in the lungs of severely ill COVID-19 patients, thus fighting the cytokine storm sometimes associated with the disease. A single fraction of low-dose radiation (1.5 Gy) was applied to both lungs, which improved the clinical situation of some patients considerably.
We looked into the technical aspects of the irradiation, and how we would treat such patients on our TrueBeams.
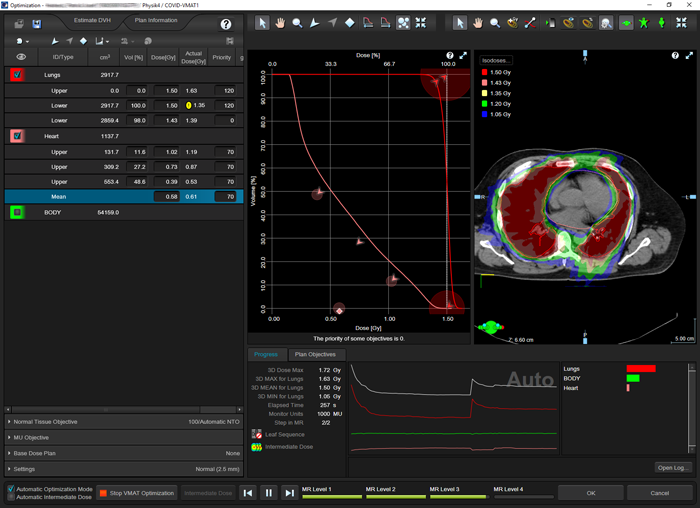
(Optimizing a single arc VMAT treatment which spares the heart.)
Fighting the Storm
For this planning study, we randomly selected ten cancer patients which received treatment in the lung region and pretended they were COVID-19 patients. The planning CT was used 'as is', although it is known that due to the disease, the lung tissue in COVID-19 patients can change ('ground glass opacities'). But we did not want to speculate whether this effect would lead to higher average lung densities.
Several questions came up immediately, for instance:
- Is it possible to set up treatment fields on our machines which are large enough?
- Is it necessary to acquire a planning CT and perform full 3D dose planning?
- If no imaging is available: is it possible to use a standard field size and standard MU?
- Is the heart a target or shall it be spared?
The last question may sound confusing. Whether an organ belongs to the PTV or the OAR group should be clear from the beginning, and in regular cancer treatments, the heart is always an organ at risk. However, the cytokine storm may also be present in the heart. In this case it would make sense to irradiate the heart as well. We will look into both options, irradiating the heart and sparing the heart.
The lungs can be treated with a broad range of techniques, ranging from simple AP/PA static fields over single ARCs (both without MLC), up to to full VMAT.
Forward planned techniques can be hand-calculated, which makes them the favorable option if there is no CT information available. On the other hand, without imaging, there is no way to spare the heart.
For the largest part of this study, we assume that the heart should be irradiated to the same extent as the lungs.
AP/PA
To address the first question regarding field size limitations on the TrueBeam, for our sample of patients we first identified the patient with the biggest lungs:
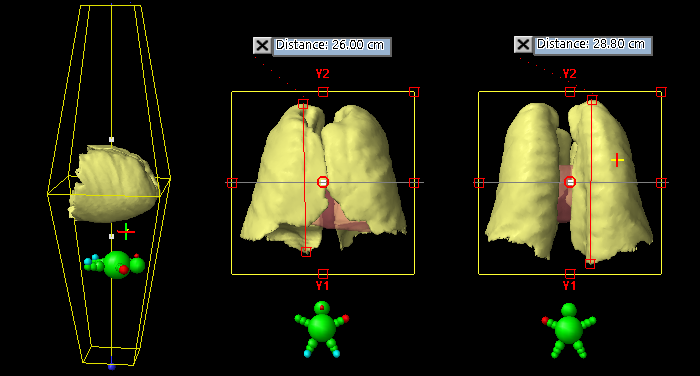
(Measuring the size of the lungs in cranio-caudal direction.)
After segmenting the organs in Eclipse with the usual tools, the plan isocenter is placed so that both lungs are well centered within square treatment fields. Now the geometry is fixed. One has to keep in mind that distances measured in Beam's-eye-view are always reported at isocenter. The consequence is that for the same lung, the measured distance beween the same two points will be larger for the 180° field than for the 0° field. The typical lung is longer on the dorsal side, which for the 180° field is closer to focus and therefore projects larger at isocenter.
The lungs pictured above, which are the largest of the sample, have a volume of 5445 cm3. However, they fit nicely inside a 32 x 32 cm field. All other patiens had smaller lungs. For simplicity, we will work with the field size of 32 x 32 cm from now on, assuming that the irradiation of some extra tissue around smaller lungs is no problem.
Calculating dose (AAA 15.6.04) and normalizing to mean lung dose of 1.5 Gy resulted in 68 MU per field1:
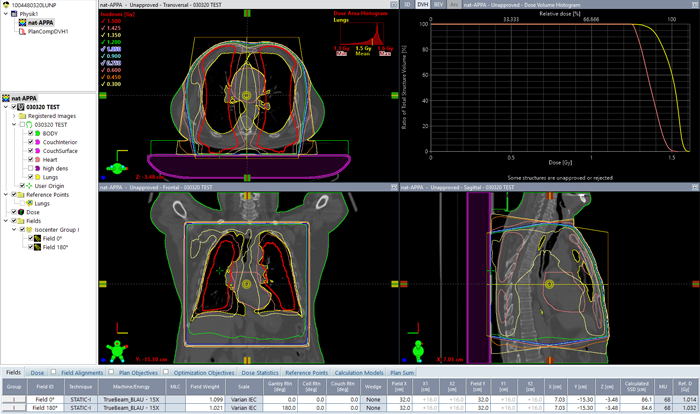
(APPA lung irradiation with 15 MV. DVH(lungs) = yellow, DVH(heart) = pink.)
The highest photon energy (15 MV in our case) was chosen for homogeneity reasons. If wedges are allowed, dose homogeneity inside the lungs could also be improved by using an enhanced dynamic wedge in the OUT direction for the 0° field, but then it is likely that the isocenter would have to be shifted to achieve the largest possible EDW field length (30 cm).
Relative to the patient, the isocenter was placed at midline, so that geometric isocenter depth for both fields was the same. Field weight was adjusted for equal MU, then the plan was again normalized to mean lung dose.
Another fine and simple option to increase homogeneity is to let the field rotate around the patient, by using the ARC technique:
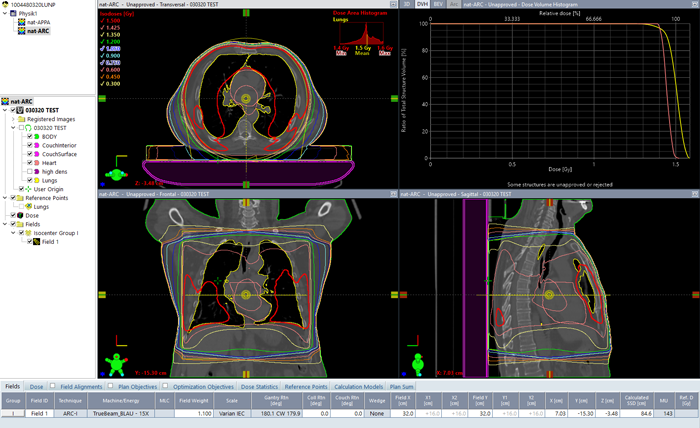
(360° ARC treatment, same field size and energy as above)
For a full 360° arc and 15 MV, 143 MU are needed to get a mean lung dose of 1.5 Gy for this patient. Dose homogeneity is significantly better compared to the AP/PA technique. Heart dose is also higher and closer to 1.5 Gy:
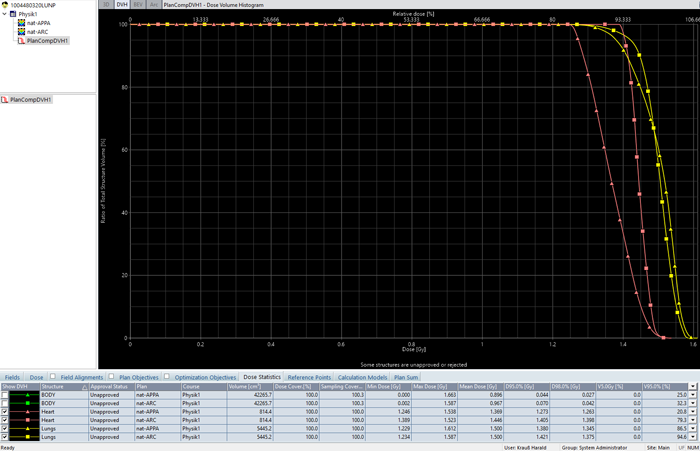
(DVH comparison: AP/PA=triangles, ARC=squares.)
Is it possible to use "Standard plans"?
The simple static techniques described above were planned on a CT data set. But what if, for some reason, no CT can be acquired? Is it possible to use the same standard plan (=MU, field size) for all patients? What dose variation between patients would we get? And if dose variation is unacceptably high, is there a way to hand-calculate MU for a mean lung dose of 1.5 Gy?
The following table contains the data for our sample of 10 patients, with the MU necessary to deliver 1.5 Gy to the lungs, and the individual deviation from average ('standard') MU. Thorax diameter was measured in AP direction along the central axis of the APPA fields. The arm position, whether they are 'up' (using some wing board) or 'down' (to both sides of the torso) will not affect the APPA MU, but they will have some (small) impact on ARC MU.

The MU variation around the average (67.5/67.5 for the AP/PA plan, 144.5 for the ARC plan) is rather small. For nine of the ten patients, it is within -4/+2% for all plans and both techniques. Here the deviation is plotted as a function of lung volume and thorax thickness:
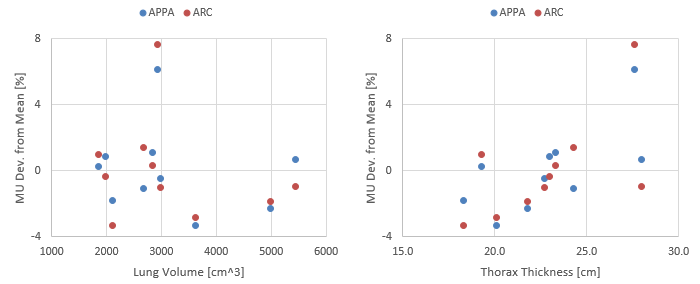
The single outlier (patient 2 with 6.2% and 7.6% deviation for APPA and ARC, respectively) is a male patient with a rather average lung volume (2918 cm3) and a rather massive upper body. Here the APPA plan:

(The most massive patient of the group requires the highest MU number for the APPA plan.)
Due to the larger fraction of normal tissue, it takes some more MU (+4.2 MU per field, to be exact) to get to the desired lung dose of 1.5 Gy.
Our sample of ten patients is rather small, and a larger patient collective would clearly improve the accuracy in the attempt to determine the machine parameters of the two alternative 'standard plans' (APPA or ARC). From 10 patients it cannot be said how representative this is. If, for instance, larger lungs would show up in the increased sample, it could even be necessary to increase the field size, up to the machine limit of 40 x 40 cm.
However, if we accept both our sample size and roughly 10% inaccuracy in lung dose, we could use standard plans with the mean MU we found, to treat COVID-19 patients.
What about hand-calculated MU?
Would it be possible to individualize the plan by using hand-calculated MU in order to improve dose accuracy?
Let's assume the patient is positioned on the treatment couch and the CAX is defined. Then the diameter of the patient can be determined with the help of couch vertical readings and the laser or ODI (optical distance indicator). This gives the isocenter depth of the APPA fields. We set up the field size of 32 x 32 cm and choose 15 MV, as above.
In water, we calculate the MU which are required for 0.75 Gy per field at isocenter. The hand calculated MU are compared to the MU from the 3D plan (1st table, column 5):
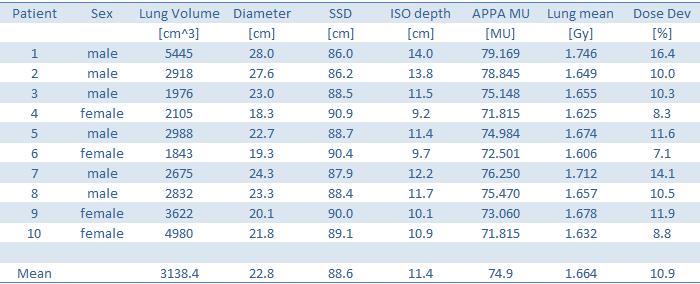
Since we have hand calculated the MU in water and disregarded lung density, a certain overdose to the lungs was expected. The amount of overdose (between 7.1 and 16.4%, last column) is larger than the variation in MU we found in the 3D plannings (-3.3 to + 6.2% relative to mean MU). Using standard plans, based on full 3D plannings of a certain patient sample seems to be a better strategy than individual hand-calculation to water.
VMAT
Let's finally assume that for some reason, for patient 1 (the one with the biggest lungs) the heart has to be spared.
The only practical solution is VMAT. But will it possible to set up such large VMAT fields on a HD-MLC? After all, the HD-MLC is optimized for stereotactic treatments (aka small fields).
We assume that treatment time is at a premium because patient condition is poor. We therefore exclude all plan setups with more than one isocenter, and allow a maximum of two arcs.
After narrowing the X-field size of both arcs a little in order to guarantee full modulation capacity, the VMAT optimization is straightforward:
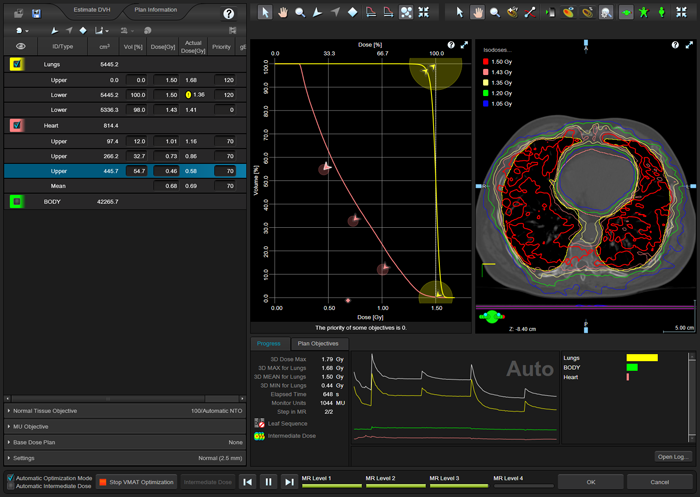
(Optimizing the plan for the patient with the biggest lungs, including heart sparing.)
Dose is calculated (AAA) and normalized to 1.5 Gy mean lung dose:
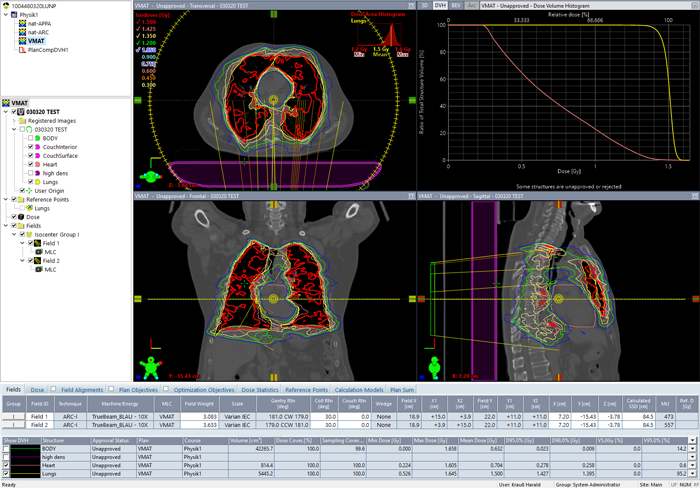
(Calculated VMAT plan with two arcs for the same patient.)
It turns out that by using VMAT, the lungs can be irradiated with the same homogeneity as with the (forward planned) ARC technique. At the same time, in the example it was possible to reduce mean heart dose to 0.7 Gy (>50% reduction).
For us, this came as no surprise. At KFJ, we treat all patients on our two (identical) TrueBeams, and the percentage of modulated plans is 90%. However, for others it may be good to know that with the HD-MLC, it is possible to cover even larger targets2.
Notes
1 Linac output calibration: 100 MU = 1 Gy at SSD100+dmax for a 10x10 field.
2 Between 2013 and 2020, there were only 12 cases where more than one isocenter had to be used to provide good target coverage.